Arsenic
David J. Vaughan – Guest Editors
Table of Contents
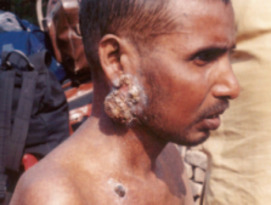
Arsenic is an element known throughout history as a classic poison. Currently, very small but highly significant concentrations of this element in drinking water supplies are causing massive health problems to many millions of people in some of the world’s poorest nations, and more localized sources related to mining and processing are also a concern. This issue of Elements will present background information on arsenic chemistry, occurrence in the Earth, production and uses, and its toxic properties.
- Arsenic
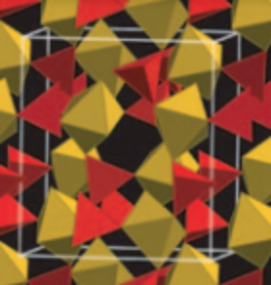
- Chemistry and Mineralogy of Arsenic
- Microbial Transformations of Arsenic in the Environment: From Soda Lakes to Aquifers
- Arsenic in Shallow, Reducing Groundwaters in Southern Asia: An Environmental Health Disaster
- Arsenic in Soils, Mine Tailings, and Former Industrial Sites
- Arsenic in Drinking Water: Impact on Human Health
Activation Laboratories Ltd. (Actlabs)
Cambridge University Press
CrystalMaker
Excalibur Mineral Corporation
Hudson Institute of Mineralogy
Meiji Techno America
PANalytical
Rigaku
RockWare
v2n3 Water on Mars
Guest editor: Harry Y. McSween Jr (University of Tennessee),
During the past several decades, spacecraft data have transformed the planets from astronomical objects into geologic worlds. Mars is the cur- rent focus of planetary exploration, and NASA’s objectives for this effort are based on the theme, “follow the water.” This issue will address new discoveries from spacecraft and from Martian meteorites about where water or ice was (or is) located and about the role of water in determin- ing the mineralogy, petrology, and geochemistry of the Martian surface.
- Water on Mars Harry Y. McSween Jr. (University of Tennessee)
- Geomorphological Evidence for Water on Mars Victor R. Baker (University of Arizona)
- The Orbital Search for Altered Materials on Mars Michael B. Wyatt (Arizona State University) and Harry Y. McSween Jr. (University of Tennessee)
- Water at the Poles and in Permafrost Regions of Mars Philip R. Christensen (Arizona State University)
- Aqueous Processes Recorded by Martian Meteorites Analyzing Martian Water on Earth Laurie A. Leshin NASA Goddard Space Flight Center) and Edward Vicenzi (Smithsonian Institution)
- Evidence for Water at Meridiani Bradley L. Jolliff (Washington University), Scott M. McLennan (Stony Brook University), and the Athena Science Team
- User Research Facilities in the Earth Sciences (February 2006 )
- Arsenic (April 2006)
- Water on Mars (June 2006)
- Early Earth (August 2006)
- Glasses and Melts: Linking Geochemistry and Materials Science (October 2006 )
- The Nuclear Fuel Cycle: Environmental Aspects (December 2006)