Gold
Robert M. Hough and Charles R. M. Butt – Guest Editors
Table of Contents
Gold fascinates researchers in many sciences. As well as being attractive as a pre cious metal, gold has important physical and electrical properties that cause it to be an ’advanced material’ for manufacturing and drug delivery in medical science. Geologically, gold can be transported in solution in ambient as well as hightem perature fluids, and its mineralogy, composition and crystallography are often used to decipher and interpret the genesis of different goldbearing ore systems. Because gold is a metal, its study requires a detailed understanding of metallography.
Why Gold is Valuable
Gold in Solution
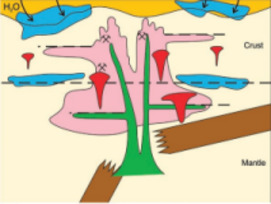
Gold Deposits: Where, When and Why
From Source to Sinks in Auriferous Magmatic-Hydrothermal Porphyry and Epithermal Deposits
The Crystallography, Metallography and Composition of Gold
The Biogeochemistry of Gold
Gold and Nanotechnology
Activation Laboratories (Actlabs)
Australian Scientific Instruments (ASI)
Earth Magazine
Excalibur
International Center for Diffraction Data (ICD)
GeoScienceWorld
Goldschmidt 2010
ioGlobal
RockWare
Savillex
SIMS workshop (Arizona State University)
Smart Elements
Society of Economic Geologists
Springer
Thermo
v5n6 Metal Stable Isotopes: Signals in the Environment
Guest editor: Thomas D. Bullen (U.S. Geological Survey) and Anton Eisenhauer
During the past decade it has been recognized that the stable isotope compositions of several metallic elements vary significantly in nature due to both biotic and abiotic processing. While this leap in our understanding has been fueled by recent advances in instrumentation and techniques in both thermal ionization and inductively coupled plasma mass spectrometry, the field of metal stable isotope geochemistry has finally moved beyond a focus on development of analytical techniques and toward using the isotopes as source and process tracers in natural and experimental systems. Often termed the “nontraditional stable isotopes,” metal stable isotope systems have found wide application in the geological, hydrological, and environmental research realms and are enjoying a rapidly expanding presence in the scientific literature. This issue of Elements will focus on several intriguing aspects of lowtemperature metal stable isotope geochemistry.
- Metal Stable Isotopes in Low-Temperature Systems: A Primer – Thomas D. Bullen (U.S. Geological Survey) and Anton Eisenhauer
- The Odds and Evens of Mercury Isotopes: Applications of Mass-Dependent and Mass-Independent Isotope Fractionation – Bridget A. Bergquist (University of Toronto) and Joel D. Blum (University of Michigan)
- Reconstructing Paleoredox Conditions through a Multitracer Approach: The Key to the Past Is the Present – Silke Severmann (University of California Riverside) and Ariel D. Anbar (Arizona State University)
- Marine Calcification: An Alkali Earth Metal Isotope Perspective – Anton Eisenhauer, Bas¸ ak Kısakürek, and Florian Böhm
- Combining Metal Stable Isotope Fractionation Theory with Experiments – Edwin A. Schauble, Merlin Méheut, and Pamela S. Hill
- Fractionation of Metal Stable Isotopes by Higher Plants – Friedhelm von Blanckenburg, Nicolaus von Wirén, Monika Guelke, Dominik J. Weiss, and Thomas D. Bullen
- Environmental and Biomedical Applications of Natural Metal Stable Isotope Variations – Thomas D. Bullen (U.S. Geological Survey) and Thomas Walczyk (University of Singapore)
- Scientific Exploration of the Moon (February 2009)
- Bentonites – Versatile Clays (April 2009)
- Gems (June 2009)
- Mineral Magmatism: From Microbes to Meteorites (August 2009)
- Gold (October 2009)
- Metal Stable Isotopes: Signals in the Environment (December 2009)
Download 2009 Thematic Preview