Graphitic Carbon
Olivier Beyssac and Douglas Rumble – Guest Editors
Table of Contents
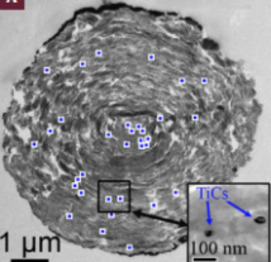
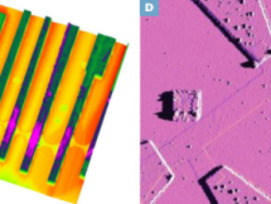
In natural systems, graphitic carbons are widespread and exhibit an infinite range of structure, from amorphous-like compounds (e.g. soots, charcoal) to crystalline graphite through a myriad of turbostratic struc- tures (e.g. coals, kerogens). A variety of structures and chemistries down to the nanometer scale control the physicochemical properties of gra- phitic carbons and determine their behavior and fate during geological processes. This issue of Elements will present recent advances in our understanding of the formation of graphitic carbons (graphitization, fluid deposition) and will discuss their role as actors and/or tracers in cosmochemistry, geobiology, geochemistry, and petrology. In particular, graphitic carbons may carry an important biological legacy in rocks, they may be used for assessing the thermal history of rocks, and they buffer the chemical composition of fluids in equilibrium with rocks. The issue will also present an introduction to the new carbon nanomaterials (e.g. graphene, carbon nanotubes), which bear structural similarities to natural graphitic carbons, and to their technological applications.
- Graphitic Carbon: A Ubiquitous, Diverse, and Useful Geomaterial
- From Organic Matter to Graphite: Graphitization
- Hydrothermal Graphitic Carbon
- Graphitic Carbons and Biosignatures
- Presolar Graphitic Carbon Spherules: Rocks from Stars
- Carbon-Based Nanoscience
AHF Analysentechnik
Australian Scientific Instruments (ASI)
Bruker Nano
Cameca
FEI
Excalibur Mineral Corporation
Geochemist’s Workbench
IAGeo Ltd
Isoprime
JEOL
Nu Instruments
Savillex
Zeiss
v11n1 MINERALOGY OF MARS
Guest Editors: John P. Grotzinger (California Institute of Technology)
The Mars Science Laboratory rover Curiosity touched down on the surface of Mars on August 5, 2012. Curiosity was built to search and explore for habitable environments. The rover has a lifetime of at least one Mars year (~23 months) and a drive capability of more than 20 km. The MSL science payload can assess ancient habitability, which requires the detection of former water, a source of energy to fuel microbial metabolism, and key elements such as carbon, sulfur, nitrogen, and phosphorus. Within 8 months of landing, we were able to confi rm full mission success. This was based on the discovery of fi ne-grained sedimentary rocks, inferred to represent an ancient lake. These rocks (Sheepbed mudstone) preserve evidence of an aqueous paleoenviron- ment that would have been suited to support a Martian biosphere founded on chemolithoautotrophy and characterized by neutral pH, low salinity, and variable redox states for both iron and sulfur species. C, H, N, O, S, and P were measured directly as key biogenic elements. The environment likely had a minimum duration of hundreds to tens of thousands of years. These results highlight the biological viability of fluvial–lacustrine environments in the ancient history of Mars and the value of robots in geologic exploration.
- Curiosity’s Mission of Exploration at Gale Crater, Mars John P. Grotzinger (California Institute of Technology) and the MSL Science Team
- Images from Curiosity: A New Look at Mars Linda C. Kah (University of Tennessee) and the MSL Science Team
- ChemCam: Chemostratigraphy by the First Mars Microprobe Roger C. Wiens (Los Alamos National Laboratory), Sylvestre Maurice (CESR, Université Paul Sabatier, Toulouse), and the MSL Science Team
- In Situ Compositional Measurements of Rocks and Soils with the Alpha Particle X-ray Spectrometer on NASA’s Mars Rovers Ralf Gellert (University of Guelph) and the MSL and MER Science Teams
- Determining Mineralogy on Mars with the CheMin X-Ray Diffractometer Robert T. Downs (University of Arizona) and the MSL Science Team
- Volatile and Isotopic Imprints of Ancient Mars Paul R. Mahaffy (Goddard Space Flight Center, NASA), Pamela G. Conrad (Goddard Space Flight Center, NASA), and the MSL Science Team
- Asteroids: Linking Meteorites and Planets (February 2014)
- Ophiolites (April 2014)
- Kaolin (June 2014)
- Unconventional Hydrocarbons (August 2014)
- Cosmogenic Nuclides (October 2014)
- Graphitic Carbon (December 2014)
Download 2015 Thematic Preview
- Mineralogy of Mars (February 2015)
- Arc Magmatic Tempos (April 2015)
- Apatite (June 2015)
- Societal and Economic Impacts of Geochemistry (August 2015)
- Supergene Metal Deposits (October 2015)
- Geomicrobiology and Microbial Geochemistry (December 2015)
Download 2015 Thematic Preview