Societal and Economic Impacts of Geochemistry
John Ludden, Francis Albarède, and Max Coleman – Guest Editors
Table of Contents
As geochemists and mineralogists, we are well aware of the impact of our science and when pushed we can often reel out great examples where our discoveries have influenced industry and the social well- being on the planet. However, this sort of drum-beating is not intuitive, and the explicit need to demonstrate impact in our science is, in many nations, being used as a measure of the required funding level for our discipline. The papers in this issue will show how we use geochemistry to achieve impact, and they will provide the basic science coupled to case studies from the hydrocarbon, mineral, environmental, and health and nutrition fields. The authors will document economic estimates of the benefits of their science; an example is the role of mass spectrometry in the oil and gas sector, in disease control, and in the use of isotopic tracers in mineral exploration.
The Impact of Geochemistry By John Ludden
Applied Geochemistry in Mineral Exploration and Mining
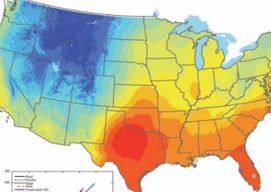
Environmental Mineralogy: New Challenges, New Materials
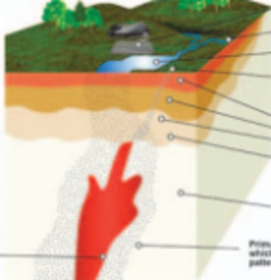
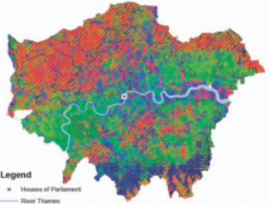
Geochemically Based Solutions for Urban Society: London, A Case Study
Stable Isotopes Trace the Truth: From Adulterated Foods to Crime Scenes
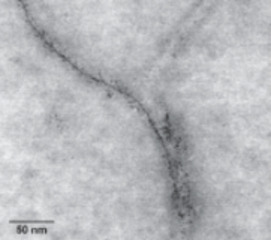
Metal Stable Isotopes in the Human Body: A Tribute of Geochemistry to Medicine
AHF Analysentechnik
Analytical Instrument Systems, Inc.
Australian Scientific Instruments (ASI)
Bruker Nano
Cambridge University Press
Cameca
Excalibur Mineral Corporation
Geochemist’s Workbench
JEOL
ProtoXRD
Rocks & Minerals
Savillex
Wiley
Zeiss
v11n5 Supergene Metal Deposits
Guest editor: Martin Reich (University of Chile) and Paulo M. Vasconcelos (University of Queensland)
Supergene metal deposits form when deeply buried orebodies are exposed at the surface and undergo oxidation, dissolution, and sig- nificant reconcentration of metals. Much of the global economic and scientific interest in these ores stems from their mineralogical diver- sity and advantages for exploitation due to their surficial development and increased grades. Supergene deposits contribute significantly to the world’s supply of metals, such as copper, aluminum, and nickel. They are also increasingly being explored and exploited as alternative sources for “critical metals,” including rare earth elements and strategic metals, which are widely used in technology and low-carbon energy applications. Furthermore, supergene metal deposits provide clues about our past climate and offer an unparalleled opportunity to explore the long-term corrosion behavior and environmental impact of natural and man-made materials. This issue of Elements will highlight some of the most recent advances in the field,including cutting-edge research in economic geology, paleoclimate and geoarcheology studies, environmental geochemistry, geobiology, and corrosion science.
- Geological and Economic Significance of Supergene Metal Deposits Martin Reich (University of Chile) and Paulo Vasconcelos (University of Queensland)
- Supergene Alteration of Ore Deposits: From Nature to Humans Harald Dill (Federal Institute for Geosciences and Natural Resources, Hannover)
- The Paleoclimatic Signatures of Supergene Metal Deposits Martin Reich (University of Chile), Paulo Vasconcelos (University of Queensland), and David Shuster (University of California, Berkeley)
- Copper Isotopic Perspectives on Supergene Processes: Implications for the Global Cu Cycle Ryan Mathur (Juniata College) and Matthew Fantle (Penn State)
- Predicting Geologic Corrosion with Electrodes Devon Renock (Dartmouth University) and Lindsay Shuller-Nickles (Clemson University)
- The Geomicrobiology of Supergene Metal Deposits Carla M. Zammit, Jeremiah P. Shuster, Emma J. Gagen and Gordon Southam (University of Queensland)
- Mineralogy of Mars (February 2015)
- Arc Magmatic Tempos (April 2015)
- Apatite (June 2015)
- Societal and Economic Impacts of Geochemistry (August 2015)
- Supergene Metal Deposits (October 2015)
- Geomicrobiology and Microbial Geochemistry (December 2015)
Download 2015 Thematic Preview