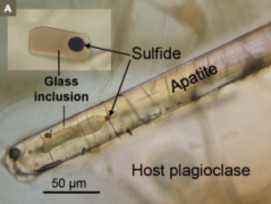
Sulfur
Charles W. Mandeville – Guest Editors
Table of Contents
This issue of Elements focuses on the geochemistry of sulfur in high-temper- ature, low-temperature, and biogeni- cally mediated processes over a wide range of scales, environments, and time intervals. Sulfur’s multiple valence states (S 2- to S 6+ ) allow for its participation in a large variety of geochemical and bio- geochemical processes. Sulfur may be one of the light elements contained in the Earth’s core and may have been crucial in core formation. Sulfur is an essential component in all life on Earth. Sulfur geochemistry continues to be used in delineating the early evolution of Earth’s atmosphere and hydrosphere, as a monitor of volcanic SO 2 and H 2 S,and as a tracer of anthropogenic sources. Recent advances in the use of multiple sulfur isotopes ( 32 S, 33 S, 34 S, and 36 S) and in situ isotopic measurements will allow sulfur stable isotopes to develop as vital tracers in the Earth and planetary sci- ences, with applications to inorganic and biogenic processes.
Touring the Biogeochemical Landscape of a Sulfur-Fueled World
Sulfur on Mars
Ancient Sulfur Cycling and Oxygenation of the Early Biosphere
Sulfur: A Ubiquitous and Useful Tracer in Earth and Planetary Sciences
Sulfur in Magmas
Ultraviolet Sensing of Volcanic Sulfur Emissions
The Arkenstone
Australian Scientific Instruments (ASI)
Bruker
Cambridge University Press
CAMECA
CrystalMaker
Dakota Matrix Minerals
Excalibur Mineral Corporation
Fugro Data Solutions
Geological Society of America
GNS Science
Rigaku
RockWare
Savillex
Thermo Scientific
v6n3 Fluids in Metamorphism
Guest editor: Bjørn Jamtveit (University of Oslo)
Fluids play a critical role during metamorphic processes. They have fi rst-order influence on both reaction kinetics and mass transfer, and thus also on the rate of metamorphism. “Volatile components,” such as H 2 O and CO 2 , may strongly influence rock rheology even in the absence of a free fluid phase. Metamorphic fluids therefore control the coupling between chemical reactions, mass transport, and defor- mation. Microstructures, compositional gradi- ents at various scales, and larger-scale deforma- tion features all reflect the dynamics of fluid–rock interactions. Moreover, the migra- tion of fluids produced during prograde meta- morphic processes or consumed during retro- gression links metamorphism with the hydrosphere, the atmosphere, and the bio- sphere. This issue sheds light on the origin of the various patterns that emerge in metamor- phic rocks as a response to changes in pressure, temperature, and the composition of pore- fi lling fluid. By following the metamorphic fluids to or from the Earth’s surface, we also aim to explain how metamorphism may affect our own environment.
- Metamorphism: From Patterns to Processes Bjørn Jamtveit (University of Oslo)
- Metamorphism: The Role of Fluids Bjørn Jamtveit (University of Oslo) and Håkon Austrheim (University of Oslo)
- Replacement Processes in the Earth’s Crust Andrew Putnis (University of Münster) and Timm John (University of Münster)
- The Mechanics of Metamorphic Fluid Expulsion James A.D. Connolly (ETH Zürich)
- Alteration of the Oceanic Lithosphere and Implications for Seafloor Processes Wolfgang Bach (University of Bremen and Woods Hole Oceanographic Institution) and Gretchen Früh-Green (ETH Zürich)
- Metamorphic Fluids and Global Environmental Changes Henrik Svensen and Bjørn Jamtveit (University of Oslo)
- Mineral Evolution (February 2010)
- Sulfur (April 2010)
- Fluids in Metamorphism (June 2010)
- Atmospheric Particles (August 2010)
- Thermodynamics of Earth Systems (October 2010)
- Sustainable Remediation of Soil (December 2010)
Download 2010 Thematic Preview