Carbon Dioxide Sequestration
David R. Cole and Eric H. Oelkers – Guest Editors
Table of Contents
The geoscientific and economic significance of the PGE is immense. Due to their extreme siderophile and chalcophile behaviour, the PGE are highly sensitive tracers of geological processses involving metal and sulfide phases. Furthermore, there are two radioactive decay series involving PGE, which combine both lithophile and chalcophile characteristics in various parent or daughter elements. PGE con- sequently offer insight into a wide range of geo- logical processes that no other group of ele- ments can provide. The PGE are also very important economically, primarily due to their “noble” character in common applications such as jewelry, electrodes, catalysts, and fuel cell technology. Unfortunately, the PGE are also bioavailable as potential toxins to organisms in the natural environment. Their widespread use, particularly in automotive catalytic converters, makes their environmental behavior a matter of increasing concern. This issue of Elements will provide an overview of our current understanding of the distribution of PGE and their isotopes in the Earth and solar system, and what this knowledge tells us about the workings of our planet, about extraction of PGE resources, and about the environmental risks attendant on their use.
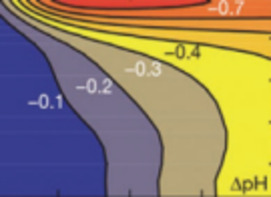
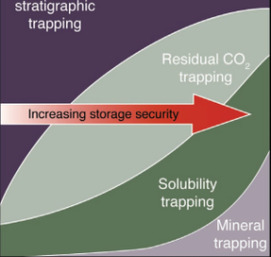
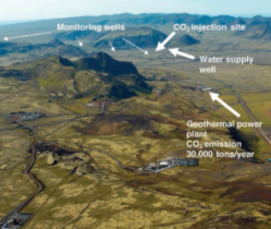
Carbon Dioxide Sequestration A Solution to a Global Problem
CO2 Capture and Transport
Ocean Storage of CO2
CO2 Sequestration in Deep Sedimentary Formations
Mineral Carbonation of CO2
Bruker AXS
Excalibur Mineral Corporation
Geological Society of London
Hudson Institute of Mineralogy
Institut national de la recherche scientifique
Meiji Techno America
Rigaku
RockWare
Smart Elements
Thermo Scientific (Part of Thermo Fisher Scientific)
University of Michigan
v4n6 Nanogeoscience
Guest editor: Michael F. Hochella, Jr.
At first glance, nano and Earth seem about as far apart as one can imagine. Nanogeo- science seems to be a word connecting oppo- sites. More specifically, a nanometer relative to a meter is the same as a marble relative to the size of this planet. But to a growing num- ber of Earth scientists, the term nanogeo- science makes perfect sense. Nanomaterials can be manufactured, but they are also nat- urally occurring. In fact, we now think that nanomaterials are essentially ubiquitous in nature. Importantly, nanomaterials often have dramatically different properties from those of the same material with larger grain size. By understanding these property changes as a function of size and shape in the nanorange, we will acquire another perspective from which to view Earth chemistry. This issue of Elements will explore our cur- rent knowledge of nanogeoscience using numerous examples from the “critical zone” of the Earth, as well as from the oceans and the atmosphere. Important insights into local, regional, and even global phenomena await our understanding of processes that are relevant at the smallest scales of Earth science studies. Nanogeoscience is at a relatively early stage of development. Therefore, large gaps in our knowledge in this area exist, making the next few years and decades an exciting time of new real- izations, discovery, and change. This issue of Elements will help promote and ener- gize this field in its early adolescence.
Nanogeoscience: From Origins to Cutting-Edge Applications Michael F. Hochella Jr.
- Structure, Chemistry, and Properties of Mineral Nanoparticles Glenn A. Waychunas and Hengzhong Zhang
- Nanoparticles in the Atmosphere Peter R. Buseck (Arizona State University) and Kouji Adachi
- Nanoparticles in the Soil Environment By Benny K. G. Theng and Guodong Yuan
- Iron Oxides as Geochemical Nanovectors for Metal Transport in Soil–River Systems Martin Hassellöv and Frank von der Kammer
- Biogenic Uraninite Nanoparticles and Their Importance for Uranium Remediation John R. Bargar, Rizlan Bernier-Latmani, Daniel E. Giammar, and Bradley M. Tebo
- Supervolcanoes (February 2008)
- Phosphates and Global Sustainability (April 2008)
- Deep Earth and Mineral Physics (June 2008)
- Platinum-Group Elements (August 2008)
- Carbon Dioxide Sequestration (October 2008)
- Nanogeoscience (December 2008)
Download 2008 Thematic Preview