Platinum-Group Elements
James M. Brenan and James E. Mungall – Guest Editors
Table of Contents
Storage of carbon in the sub- surface involves introduction of supercritical CO2 into rock formations beneath the sur- face of the Earth, typically at depths of 1000 to 4000 meters. Although CO2 is a relatively benign substance, the volume being considered is large. If developed to its envisaged potential, geologic sequestra- tion will entail the pumping of CO2 into the ground at roughly the rate we are extracting petroleum today. To have the desired impact on the atmospheric carbon budget, CO2 must be effi- ciently retained underground for hundreds of years. Any underground storage sys- tem will have to account for the natural characteristics of subsurface formations; some are advantageous for storage while others are not. When foreign materials are emplaced in subsurface rock formations, they change the chemical and physical environment. Understanding and predicting these changes are essential for deter- mining how the subsurface will perform as a storage container. The specific scien- tific issues that underlie sequestration technology involve the effects of fluid flow combined with chemical, thermal, mechanical, and biological interactions between fluids and surrounding geologic formations. Complex and coupled interactions occur both rapidly as the stored material is emplaced underground, and gradually over hundreds to thousands of years. The long sequestration times needed for effec- tive storage and the intrinsic spatial variability of subsurface formations provide challenges to both geoscientists and engineers. A fundamental understanding of mineralogical and geochemical processes is integral to this success.
The Platinum-Group Elements: “Admirably Adapted” for Science and Industry
- Platinum-Group Elements in Cosmochemistry
- Applications of PGE Radioisotope Systems in Geo- and Cosmochemistry
- Platinum-Group Elements: A New Set of Key Tracers for the Earth’s Interior
- Ore Deposits of the Platinum-Group Elements
- Environmental Relevance of the Platinum-Group Elements
Activation Laboratories Ltd (Actlabs)
Bruker AXS
CAMECA
Excalibur Mineral Corporation
Hudson Institute of Mineralogy
Meiji Techno
Rigaku
RockWare
Smart Elements
Terra Publishing
Thermo Fisher Scientific
v4n5 Carbon Dioxide Sequestration
Guest editor: David R. Cole (Oak Ridge National Laboratory) and Eric H. Oelkers (LMTG-Université de Toulouse)
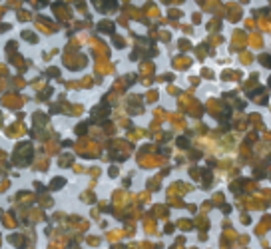
The geoscientific and economic significance of the PGE is immense. Due to their extreme siderophile and chalcophile behaviour, the PGE are highly sensitive tracers of geological processses involving metal and sulfide phases. Furthermore, there are two radioactive decay series involving PGE, which combine both lithophile and chalcophile characteristics in various parent or daughter elements. PGE con- sequently offer insight into a wide range of geo- logical processes that no other group of ele- ments can provide. The PGE are also very important economically, primarily due to their “noble” character in common applications such as jewelry, electrodes, catalysts, and fuel cell technology. Unfortunately, the PGE are also bioavailable as potential toxins to organisms in the natural environment. Their widespread use, particularly in automotive catalytic converters, makes their environmental behavior a matter of increasing concern. This issue of Elements will provide an overview of our current understanding of the distribution of PGE and their isotopes in the Earth and solar system, and what this knowledge tells us about the workings of our planet, about extraction of PGE resources, and about the environmental risks attendant on their use.
Carbon Dioxide Sequestration A Solution to a Global Problem Eric H. Oelkers (LMTG-Université de Toulouse) and David R. Cole (Oak Ridge National Laboratory)
- CO2 Capture and Transport Edward S. Rubin
- Ocean Storage of CO2 E. Eric Adams and Ken Caldeira
- CO2 Sequestration in Deep Sedimentary Formations Sally M. Benson and David R. Cole (Oak Ridge National Laboratory)
- Mineral Carbonation of CO2 Eric H. Oelkers (LMTG-Université de Toulouse), Sigurdur R. Gislason and Juerg Matter
- Supervolcanoes (February 2008)
- Phosphates and Global Sustainability (April 2008)
- Deep Earth and Mineral Physics (June 2008)
- Platinum-Group Elements (August 2008)
- Carbon Dioxide Sequestration (October 2008)
- Nanogeoscience (December 2008)
Download 2008 Thematic Preview