Greenalite: A Template Fit for Life?
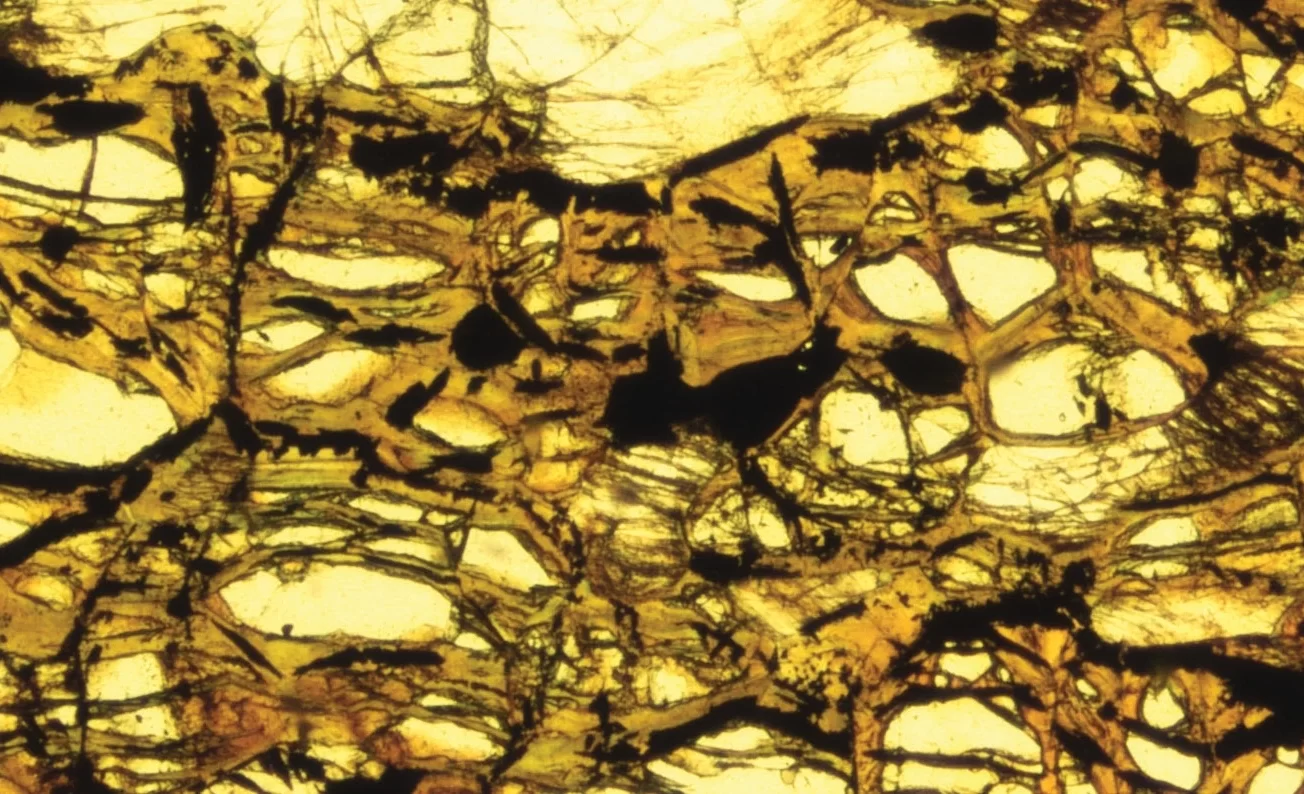
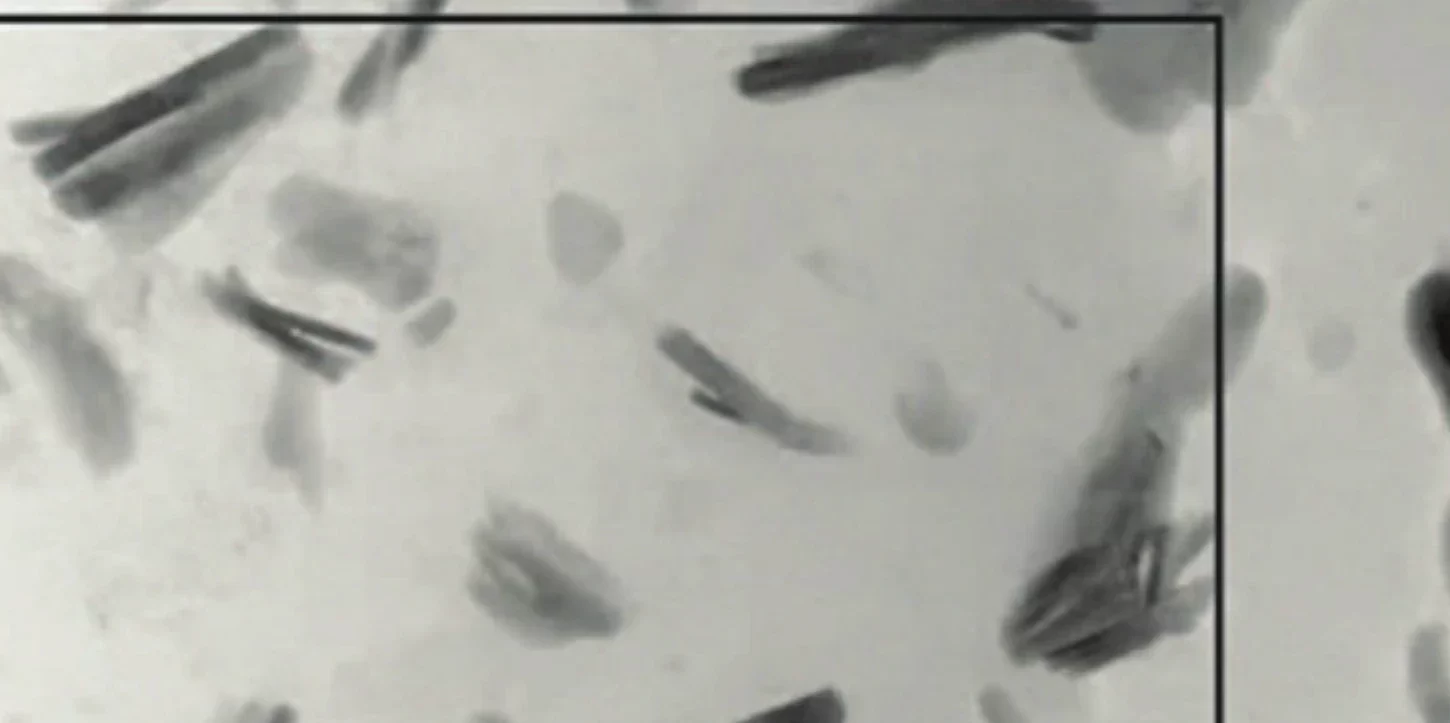
Clays have long been implicated in the story of life’s origin. This idea gained support when experiments suggested that tiny crystals of acid-preactivated montmorillonite catalyze the growth of prebiotic polymers. From a geological viewpoint, there are good reasons to consider another clay—greenalite (Fe₃Si₂O₅(OH)₄). Model predictions and observations from ancient sedimentary rocks indicate that nanoparticulate greenalite was a major phase produced during hydrothermal venting in ancient oceans and lakes. Greenalite is an iron-rich, redox-active mineral whose modulated crystal structure provides surfaces with repetitive, parallel grooves of the right size and orientation to align and potentially facilitate the assembly of long, linear biopolymers, thereby addressing a significant challenge for prebiotic chemistry—the synthesis of polymers with genetic and catalytic functions essential for life.
Greenalite: A Template Fit for Life? Read More »