December 2023 Issue Table of Contents
There is no doubt that mineralogy, texture, and microfabric, as primary characteristics of an ore, affect mineral processing operations. Their direct effects on extractive metallurgical processes and the associated optimization potential, however, are less well documented. Here, we examine the status of geometallurgical approaches in extractive metallurgy by focusing on the effects of primary ore characteristics in hydrometallurgical and pyrometallurgical processes. Two selected case studies illustrate the linkages. Using quantitative data analytics on ores and concentrates, the possibilities for optimized and sustainable metal extraction and waste valorization are discussed.
1811-5209/23/0019-0365$2.50 DOI: 10.2138/gselements.19.6.365
Keywords: Hydrometallurgy; pyrometallurgy; sustainability; Witwatersrand gold fields; Kalahari manganese deposit
INTRODUCTION
Extractive metallurgy, unlike mineral processing, generally aims at extracting and purifying specific elements from ores or mineral concentrates by inducing suitable chemical changes (Dutta et al. 2018). Hydrometallurgy relates to the chemical leaching of elements into, and their recovery from, an aqueous solution (an analogous process to hydrothermal mineralization). Pyrometallurgy, on the other hand, relates to the high-temperature treatment of ores and mineral concentrates, either in the solid or liquid state (akin to metamorphic and igneous processes, respectively), to concentrate the element(s) of interest into separate phase(s).
At first sight, it may appear obvious that geometallurgy should focus on the link between primary ore characteristics and mineral processing outcomes as minerals are preserved during mineral processing operations (Pereira et al. 2023 this issue; Frenzel et al. 2023 this issue), but destroyed in extractive metallurgy. However, primary ore characteristics will also impact the performance of extractive metallurgy operations. This is particularly important where ores are subjected to direct chemical treatment with minimal or no prior mineral concentration, as is common for some ferrous metal ores (e.g., Fe, Cr, and Mn), as well as other ore-types (e.g., some Cu, Au, and U ores). Some ore characteristics may also be inherited by mineral concentrates, and this can affect subsequent extraction processes, despite the concentrates meeting certain physical and chemical specifications for downstream processing.
Whether extractive metallurgy is applied directly or follows mineral processing, full optimization of the entire value chain from ore body to refined product is only possible if the effects of primary ore characteristics on all stages of the chain are well integrated and understood. This contribution outlines the current efforts to extend geometallurgy to extractive metallurgy operations. The relevance and impacts of the approach are examined in two case studies.
HYDROMETALLURGY
In hydrometallurgy, the process of leaching raw materials can be classified into four broad categories: in-situ leaching, percolation leaching, tank/vat leaching, and agitated leaching. In-situ leaching extracts metals directly from ores that remain in the ground (Habashi 1999). These ores must have sufficient permeability, a mineralogy amenable to leaching, and a geological context suited to guide and contain the leaching solutions (lixiviants). Percolation, or heap leaching, typically involves allowing a suitable lixiviant to seep into and through a mass of crushed ore at the surface. Tank and vat leaching involve placing milled and sized ore (or mineral concentrates) into large tanks or vats containing the lixiviant at suitable conditions (e.g., ambient or elevated pressure and temperature) to leach the valuable metals from the ore into the aqueous solution. Agitation leaching is a modification of tank/vat leaching, used for very fine particles that require agitation in a slurry with the lixiviant in order to extract metals. In all cases, the pregnant (i.e., metal-containing) solution is treated further through processes, such as solvent extraction and selective precipitation, to recover the valuable metals. Mineralogy and microfabric no longer play a role in these latter processes, although the primary ore will influence the chemical composition of the pregnant solution. The solid leach residues, by contrast, are essentially chemically modified primary ores.
Leaching processes are affected by many of the same primary ore characteristics as mineral processing operations (Table 1). Of particular importance are mineral grain sizes and associations, ore and gangue mineralogy and mineral abundances, as well as mineral liberation in the crushed material. An understanding of ore type and valuable metal distribution/liberation is thus essential for predictive modelling purposes in leaching operations. The extractive metallurgy of gold provides typical examples for all principal types of leaching, as well as the critical influence of primary ore characteristics. Based on gold deportment, mineral association, liberation and microfabric, and the resulting process behavior, three principal types of gold ores may be distinguished (Habashi 1999):
- Free-milling gold ores mostly contain native gold (or electrum) that is easily liberated during milling operations, with >90% of Au recoverable by conventional tank/vat leaching.
- Refractory gold ores mostly contain gold as submicroscopic grains or atomic-scale substitutions (sometimes misleadingly called “invisible”) in minerals such as arsenopyrite or pyrite. Such ores require excessive reagent usage and/ or additional pre-treatment processes, such as roasting (a pyrometallurgical process; see below), to recover an economically feasible amount of gold.
- Complex gold ores from which high gold recoveries are possible, but only under modified or more intense leaching conditions due to the presence of deleterious elements and minerals.
The following case study illustrates the importance of geometallurgical knowledge for the successful leaching of gold in the world’s largest gold province, the Witwatersrand gold fields in South Africa.
OPTIMIZATION OF GOLD RECOVERY FROM WITWATERSRAND ORES
With more than 52,000 t of gold produced in almost 140 years of mining and more than 30,000 t remaining in geological resources, the Witwatersrand gold fields constitute the world’s largest gold province (Fig. 1; Frimmel and Nwaila 2020). The Witwatersrand ore bodies (referred to as “reefs”) are bound to quartz-pebble conglomerate horizons of Archean age that can often be correlated across distances of >300 km (Minter et al. 1993). Most of the Witwatersrand gold occurs in two sedimentary facies: thin pebble lags (residual accumulation of surface rock fragments after removal of finer material by winds) representing aeolian deflation surfaces, often associated with kerogen (carbonaceous matter) seams; and conglomeratic, high-energy fluvial channel deposits (Frimmel 2005). Whilst the richest ore bodies were decimeter-thick conglomerates with grades of >20 g/t Au, mined reefs currently contain between 3–9 g/t Au. Uranium (100–350 g/t U) is an important by-product in some operations.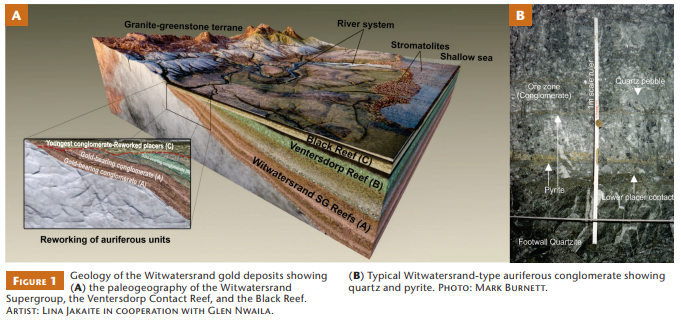
Gold-bearing quartz-pebble conglomerates of the Witwatersrand Basin usually contain quartz, pyrite, and sheet silicates (pyrophyllite, mica, chlorite), with variable amounts of kerogen, other sulfides (e.g., arsenopyrite, chalcopyrite, pyrrhotite), and many trace minerals (e.g., chromite, zircon) (e.g., Hallbauer and Maske 1986). Native gold (free-milling) is the most important carrier of gold, whereas uranium occurs mostly in uraninite and more complex oxide minerals (e.g., brannerite). Although the Witwatersrand gold ores share many general mineralogical and chemical characteristics, localized variations exist. For example, some ores contain a high proportion of solid kerogen, while others have a high proportion of minerals related to post-depositional hydrothermal overprints (e.g., pyrrhotite, chalcopyrite).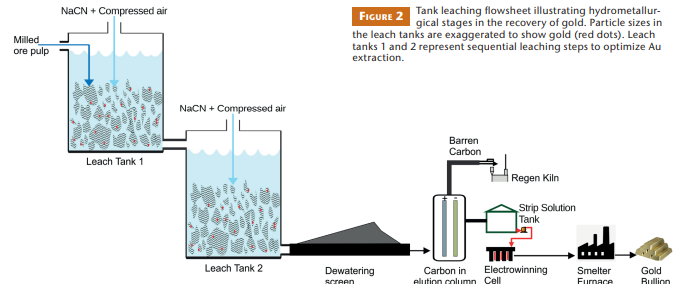
Gold is usually recovered from the Witwatersrand ores using a conventional cyanide-based tank leaching process (Fig. 2). Recovery is challenged by the fine grain size of the native gold (<100 μm; Hallbauer and Maske 1986), its close association with reactive sulfide minerals (e.g., pyrite, arsenopyrite), and the abundance of kerogen. These challenges are addressed by selective mining to separate ores based on their mineralogical composition, fine milling to achieve surface liberation of the gold, and varying the concentration of reagents and hydrometallurgical plant configuration (cf. Habashi 1999).
The process of blending (i.e., mixing of ores extracted from different reefs) is indispensable for extending the life-ofmine of large-scale gold operations in the Witwatersrand gold fields. Downstream processing must then be able to adapt to such blended ores. The benefits of blending include: (a) enhanced extraction efficiency, (b) lower reagent consumption, (c) reduced feed variability, and (d) recovery of valuable metals from low-grade ores blended with high-grade counterparts.
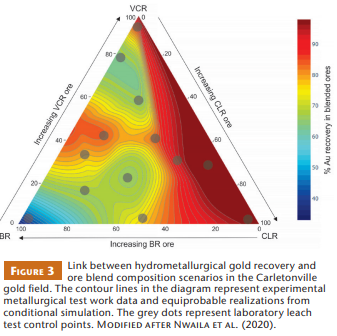
An example of gold mining operations where ores with different primary properties are blended is the Driefontein mining complex in the Carletonville gold field, where one gold cyanidation plant processes ores from four different reefs: the Black Reef (BR), Carbon Leader Reef (CLR), Middelvlei Reef (MR), and Ventersdorp Contact Reef (VCR). Based on this context, Nwaila et al. (2020) combined quantitative data on ore properties with experimental observations concerning the performance of the hydrometallurgical process route. Diagnostic leaching tests involving blends with various proportions of ores extracted from the BR, CLR, and VCR were conducted to optimize gold recovery. The interactions of the three ores were also recorded in terms of mineralogy, gold deportment, leaching rates, and Au recovery.
A ternary diagram with the three studied ore types as end members illustrates the expected gold recoveries depending on ore blend composition (Fig. 3). Ore blends with high contents of kerogen and trace amounts of copper and arsenic (i.e., high BR proportions) yield lower gold recovery rates. In contrast, high Au recovery is expected when CLR and VCR dominate the blend (Fig. 3). This is due to the high degree of Au liberation and the presence of leachable kerogen in the CLR. Consequently, mineralogy and texture strongly determine the feasibility of blending. These results, as summarized in Figure 3, are currently applied at Driefontein to minimize gold losses associated with ore blending. Modelling of intrinsic ore variation relative to reagent concentration or pressure– temperature leach conditions may further enhance Au recovery outcomes.
PYROMETALLURGY
Chemical changes in the pyrometallurgical treatment of ores and concentrates occur at high temperatures, ranging from 100 to 3000°C (Dutta et al. 2018). Various processes are applied, depending on the products required. Roasting, for example, may be used to convert sulfide minerals to oxide phases more amenable to hydrometallurgical treatment. Direct smelting of sulfide-rich mineral concentrates (e.g., from flotation) produces a matte (sulfide-melt containing, e.g., Cu, Ni) and a slag (oxide-melt containing unwanted residues). The matte is then exposed to further pyrometallurgical and/or hydrometallurgical treatment to produce refined metals (e.g., Cu, Ni, Pt).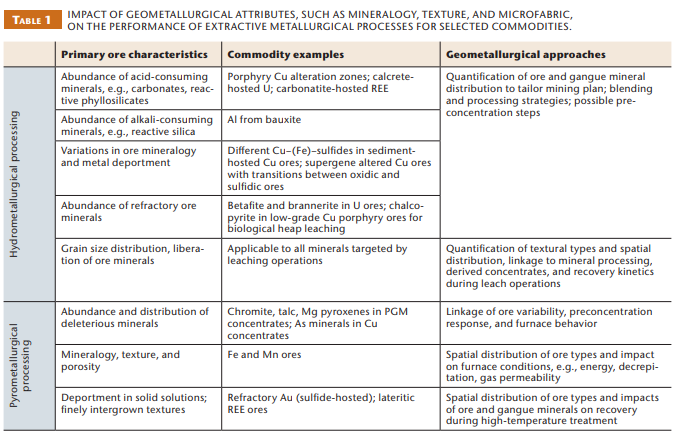
 For some ferrous metals (e.g., Fe, Mn, Cr), the ore is mixed with a reductant, typically coking coal, and fluxes, like dolomite and quartz, to reduce the oxidic ore minerals to a metal alloy and promote the formation of a slag. Solidstate pre-reduction in the furnace first often produces divalent oxides. Subsequent melting and reduction of these divalent oxides forms an alloy, which can be separated from the slag. In some instances, the commodity of interest is captured by the slag for further processing (e.g., Ti from ilmenite smelting). Lastly, volatiles and dust are produced in pyrometallurgical processes and may be a source of by-products (e.g., sulfuric acid in Cu smelting).
For some ferrous metals (e.g., Fe, Mn, Cr), the ore is mixed with a reductant, typically coking coal, and fluxes, like dolomite and quartz, to reduce the oxidic ore minerals to a metal alloy and promote the formation of a slag. Solidstate pre-reduction in the furnace first often produces divalent oxides. Subsequent melting and reduction of these divalent oxides forms an alloy, which can be separated from the slag. In some instances, the commodity of interest is captured by the slag for further processing (e.g., Ti from ilmenite smelting). Lastly, volatiles and dust are produced in pyrometallurgical processes and may be a source of by-products (e.g., sulfuric acid in Cu smelting).
Pyrometallurgical treatment is of particular importance for the production of iron, ferroalloys, and steel from oxide ores. High-grade ores of Fe, Mn, and Cr require little to no prior mineral processing. Nevertheless, some ore characteristics have an important influence on the efficiency of pyrometallurgical treatment (Table 1):
- Ore texture, including porosity, influences the breakage behavior of the ore, and hence the particle size distribution of the furnace feed. Particle sizes of furnace feed are typically coarser than 6 mm, referred to as lumpy ore, with fines being <6 mm. Fines require sintering (process subjecting aggregate material to high temperature and pressure to compact the loose material into a solid object) or pelletization (method of agglomeration, or particle size enlargement, in which material fines are processed into pellets or granules) prior to smelting. The minimization of fines is therefore important from a resource utilization and energy efficiency perspective.
- Mineralogy. The presence of hydrous minerals or carbonates causes volatile loss on heating. Where their modal abundance is of significance, treatment must be undertaken prior to furnace processes. Volatiles are ideally removed through sintering, as implemented in the processing of carbonate-rich Mn ores. This has the added advantage of an upgraded sinter product through residual enrichment of the element of interest and coarsening the particle size. Sintering also improves resistance to abrasion and fines generation during transport. The occurrence and abundance of OH- and H2O-bearing ore minerals, such as goethite (FeOOH), must be monitored, as these can release water in the furnace, and thereby create fines through physical breakage from volatile release.
In the following case study, mainly mineralogy is considered to illustrate its impact on energy consumption in the pre-reduction of high-grade Mn ores from the Kalahari manganese deposit (KMD) in South Africa.
IMPACT OF ORE VARIABILITY IN THE KMD ON FURNACE ENERGY CONSUMPTION
The KMD accounts for 78% of global land-based Mn resources, making South Africa the world’s leading producer of Mn (~37% of global production). Manganese ore in the KMD occurs as three distinct sedimentary layers intercalated with banded iron formations. Together, these comprise the Paleoproterozoic Hotazel Formation of the Transvaal Supergroup (Chetty 2008). Three main ore types occur in the KMD: sedimentary-diagenetic ores (low grade, ~38 wt.% Mn), hydrothermally altered ore (high grade, >45 wt.% Mn), and supergene ores (intermediate and high grade, 38–45 wt.% Mn). The low-grade ores occur throughout the KMD, accounting for 97% of the total resource. The highgrade ores are restricted to the northern part of the deposit, and account for <3% of the resource, with the supergene ores constituting <1%, and geographically restricted to the southeastern margin of the deposit. The three ore types are mineralogically and texturally distinct. Braunite (Mn2+Mn3+6O8(SiO4)) and kutnahorite (Ca(Mn2+,Mg2+) (CO3)2) predominate as ore minerals in the low-grade sedimentary-diagenetic ores, whereas hausmannite (Mn2+(Mn3+,Fe3+)2O4), braunite II (Ca(Mn3+,Fe3+)14SiO24), and bixbyite ((Mn3+,Fe3+)2O3) are key minerals in the high-grade hydrothermally-altered ores. The intermediate- to high-grade supergene ores comprise todorokite ((Na,Ca,K)2(Mn4+,Mn3+)6O12·3-4.5(H2O)), cryptomelane (K(Mn4+,Mn3+)8O16), and pyrolusite (Mn4+O2).
This case study focuses on the high-grade hydrothermally-altered ores, as these show the most complex and variable mineral assemblages with more than 100 minerals reported. Infiltration of hydrothermal fluids along north– south striking faults caused extensive alteration of the sedimentary-diagenetic protolith, forming high-grade ore through carbonate dissolution and recrystallization (Chetty 2008).
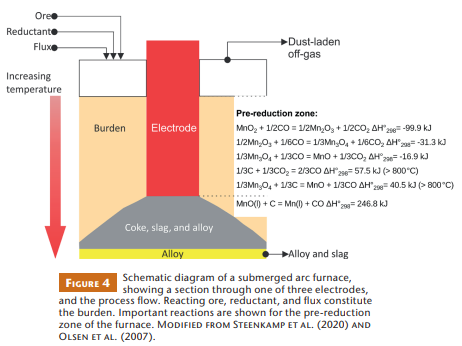 For pyrometallurgical processing, the high-grade manganese-oxide ores of the KMD are combined with flux and coke and fed into a submerged arc furnace (SAF). This furnace contains three equidistant electrodes submerged in the burden of reacting raw materials, as well as a coke, slag, and alloy bed in its lower part (Fig. 4). Electrical energy is dissipated into the material through a combination of arcing and resistive heating of the coke–slag–alloy bed (Steenkamp et al. 2020). The price of electricity is a key cost driver for SAF smelting of the Mn ores, accounting for 45%–50% of smelter costs. Energy consumption, which is mostly influenced by solid-state reactions in the pre-reduction zone of the SAF, therefore becomes an important parameter to minimize. Figure 4 shows the key Mn oxide reactions that take place between 200 and 800 °C, all of which are exothermic. Ideally, the final reaction of MnO to Mn should be the only endothermic reaction. However, above 800°C, the reduction of Mn3O4 to MnO also becomes endothermic because of the simultaneous highly endothermic reaction of CO2 with solid C (the Boudouard Reaction; Olsen et al. 2007), which greatly reduces energy efficiency.
For pyrometallurgical processing, the high-grade manganese-oxide ores of the KMD are combined with flux and coke and fed into a submerged arc furnace (SAF). This furnace contains three equidistant electrodes submerged in the burden of reacting raw materials, as well as a coke, slag, and alloy bed in its lower part (Fig. 4). Electrical energy is dissipated into the material through a combination of arcing and resistive heating of the coke–slag–alloy bed (Steenkamp et al. 2020). The price of electricity is a key cost driver for SAF smelting of the Mn ores, accounting for 45%–50% of smelter costs. Energy consumption, which is mostly influenced by solid-state reactions in the pre-reduction zone of the SAF, therefore becomes an important parameter to minimize. Figure 4 shows the key Mn oxide reactions that take place between 200 and 800 °C, all of which are exothermic. Ideally, the final reaction of MnO to Mn should be the only endothermic reaction. However, above 800°C, the reduction of Mn3O4 to MnO also becomes endothermic because of the simultaneous highly endothermic reaction of CO2 with solid C (the Boudouard Reaction; Olsen et al. 2007), which greatly reduces energy efficiency.
The various Mn oxides present in the KMD (Fig. 4) may be approximated by simple minerals such as pyrolusite (Mn4+O2), bixbyite (Mn3+2O3), and hausmannite (Mn2+Mn3+2O4). Because Mn has different oxidation states, the reduction of these minerals will release/consume very different amounts of energy (Fig. 4). Furthermore, the reactivity of minerals in the pre-reduction zone appears to be dependent on the type of minerals present. Tangstad et al. (2001) found that ores with tetravalent Mn minerals reduced faster than those with divalent and trivalent Mn minerals, although details were lacking on the quantitative mineralogy of the ores tested. Kinetic behavior will influence the degree to which Mn3O4 is reduced before the Boudouard reaction begins and, therefore, will influence energy consumption.
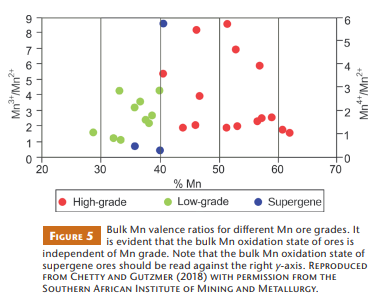 Considering the possible relationship between Mn grade and bulk oxidation state, Chetty and Gutzmer (2018) studied the modal mineralogy of drill cores from different parts of the northern KMD, as well as some supergene ores from the southeastern margins. Modal mineralogy and mineral chemistry data were combined to calculate bulk assays for validation purposes. Considering Mn valence states and their partitioning in the minerals, the total contents of divalent, trivalent, and tetravalent Mn of the studied ores were determined. Figure 5 shows that the Mn grade of ores from the KMD does not covary with bulk Mn oxidation state. Different bulk mineral assemblages can reflect very similar bulk oxidation states. For example, most high-grade hausmannite-rich ores display virtually identical bulk Mn oxidation states to low-grade braunite– kutnahorite-rich ores. This result emphasizes the need to understand the mineralogy, not only the grade or total Mn-associated oxygen, to optimize energy efficiency in the reduction of the manganese ores of the KMD.
Considering the possible relationship between Mn grade and bulk oxidation state, Chetty and Gutzmer (2018) studied the modal mineralogy of drill cores from different parts of the northern KMD, as well as some supergene ores from the southeastern margins. Modal mineralogy and mineral chemistry data were combined to calculate bulk assays for validation purposes. Considering Mn valence states and their partitioning in the minerals, the total contents of divalent, trivalent, and tetravalent Mn of the studied ores were determined. Figure 5 shows that the Mn grade of ores from the KMD does not covary with bulk Mn oxidation state. Different bulk mineral assemblages can reflect very similar bulk oxidation states. For example, most high-grade hausmannite-rich ores display virtually identical bulk Mn oxidation states to low-grade braunite– kutnahorite-rich ores. This result emphasizes the need to understand the mineralogy, not only the grade or total Mn-associated oxygen, to optimize energy efficiency in the reduction of the manganese ores of the KMD.
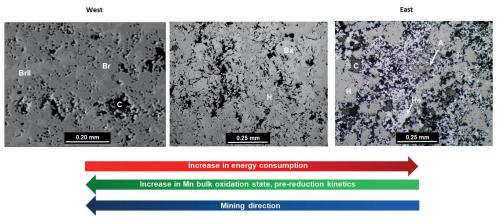
It is important to note that the bulk Mn oxidation states for high-grade ores of the northern part of the KMD show a broad systematic variation in space, with lower ratios exhibited by hausmannite-rich ores that are more prominent in the east, and higher ratios displayed by braunite II–bixbyite ores that dominate in the west (Fig. 6). This distribution has been tentatively linked to fluid–rock ratios during hydrothermal alteration (Chetty 2008). For pyrometallurgical processing, this geologically controlled distribution can be used to predict possibly faster pre-reduction and lower energy consumption in the SAF as mining progresses westward in the northern KMD. Furnace conditions should be adapted accordingly, as these may oversupply electricity to drive reactions that are more exothermic than for previously mined high-grade ores.
As noted already, ore texture and porosity are important drivers for ore breakage characteristics during mining and ore handling. Given the changes in mineralogy, as well as variable porosity imparted to the ores during hydrothermal alteration in the northern KMD, quantitative texture and porosity assessment should be conducted to link mineral assemblages with ore hardness and strength properties, as well as energy consumption, to provide a holistic integration of variables and their impacts on smelting with continued mining. Consequently, operational flexibility can be implemented to optimize Mn alloy production and resource utilization.
GEOMETALLURGY AND SUSTAINABILITY
To assess the sustainability of extractive metallurgy operations, the following parameters must be considered: reagents, water, energy, recoveries of valuables in product and by-product streams, waste generation, environmental impact of residues, and emissions. The above examples illustrate how geometallurgical approaches can positively impact some of these parameters and, thus, the sustainability of extractive metallurgy operations.
The minimization of reagent consumption in leaching is important for operational expenditure. In a geometallurgical context, ore variability must be understood to manage this parameter. The recycling of reagents is also important for process efficiency in developing leaching and extraction flowsheets, particularly in arid regions where water is scarce, and remote areas where reagent transport becomes prohibitively expensive. More environmentally friendly reagents may also be sought, such as alternatives to cyanide for gold leaching. In all these endeavors, the entire life cycle of the reagents and associated impacts must be considered, and all must feature in a geometallurgical model.
Pyrometallurgical processes are often carbon and energy intensive, as in the production of ferroalloys, iron, and steel. There is a strong drive toward decarbonizing the industry. Reductants with low carbon footprints, such as biomass and green hydrogen (generated using renewable energy sources), are gaining momentum as useful alternatives. A wide range of possible technological approaches already exists for iron production using hydrogen as a reductant (Souza Filho et al. 2022). There are also efforts to incorporate renewable energy into pyrometallurgical processes, such as solar thermal heating of feed and product streams (e.g., Hockaday et al. 2020) or the use of solar electricity to power furnaces. Energy efficiency can be further increased by heat recovery from furnace off-gas streams and slags for electricity generation. Understanding feed variability will help to optimize the impact of such initiatives.
Pyrometallurgical processes are further characterized by the generation of slags, which are generally discarded as waste. However, this leads to the loss of incompletely reduced metal oxides and metals entrained in the slag. Consequently, slag reprocessing is common, particularly for the recovery of high-value metals like cobalt (Jones and Pawlik 2019). From a circular economy viewpoint, slags should be considered by-products, and during process design, conditioned slags should be produced for further treatment, whether for metal reclamation or utilization of the slag itself, e.g., as construction material. Quantitative characterization of the slags and other by-product streams is vital in all waste valorization approaches toward sustainable processing, as the phases, crystallinity, porosity, and texture of the slag are the main factors that dictate the viability of potential products. Well-understood ore characteristics and good knowledge of their interactions with other raw materials in the furnace, complemented by advances in thermochemical modelling, offer an opportunity for predictive slag design to enable further use as a by-product.
Given the steady depletion of grades in increasingly complex primary ores available for extraction, as well as associated higher mining and processing costs, efficient utilization of secondary resources will be required to meet future metal demand. The generation of increasing volumes of tailings is of similar relevance and requires consideration for environmental and socio-economic risk mitigation, as well as value extraction (Parbhakar-Fox and Baumgartner 2023 this issue). The recycling of end-of-life products, sometimes referred to as urban mining, is also characterized by complex, variable, and rapidly growing waste streams that may pose environmental threats, but have large potential value. A notable example is e-waste. Integrated flowsheets for the physical beneficiation and metallurgical treatment of these complex materials— informed by a geometallurgical approach to material characterization and predictive process modelling—will be required for feasible value reclamation.
OUTLOOK
This paper used two well-documented geological examples to provide an indication of the inherent potential of geometallurgical approaches in extractive metallurgy. Yet, in practice, glaring gaps remain between the different knowledge silos along the value chain from exploration to metal extraction and recovery. Geometallurgy can help to bridge these gaps to realize the full value of different raw materials, and design integrated flowsheets to maximize benefit in complex process chains. As this issue of Elements aims to bring awareness of geometallurgy, it is prudent to close with a consideration of how the concept may be better incorporated into postgraduate curricula. Simulation-based approaches would likely be best suited to teach troubleshooting skills and critical thinking through gamification and modelling. Corresponding modules may be integrated into economic geology, as well as geological, mining, and metallurgical engineering curricula. This can help to build a new generation of professionals in this decidedly interdisciplinary field.
ACKNOWLEDGMENTS
The authors wish to thank Mintek and the Wits Mining Institute at the University of the Witwatersrand for funding the presented case studies. Dr. Ajay Patil and an anonymous reviewer are thanked for their constructive reviews of the manuscript. The guest and chief editors are also gratefully acknowledged for their inputs to the manuscript. Mintek is thanked for funding open access to this article.
REFERENCES
Chetty D (2008) A Geometallurgical Evaluation of the Ores of the Northern Kalahari Manganese Deposit, South Africa. PhD thesis, University of Johannesburg, 271 pp
Chetty D, Gutzmer J (2018) Quantitative mineralogy to address energy consump-tion in smelting of ores from the Kalahari Manganese Field, South Africa. In:
Jones RT, den Hoed P, Erwee MW (eds) Proceedings: Infacon XV: International Ferro-Alloys Congress, Southern African Institute of Mining and Metallurgy, Cape Town, pp 25-28
Dutta SK, Lele AB, Choksi YB (2018) Extractive Metallurgy, Processes and Applications. PHI Learning Private Limited, 169 pp
Frenzel M, Baumgartner R, Tolosana-Delgado R, Gutzmer J (2023) Geometallurgy: present and future. Elements 19: 345-351
Frimmel HE (2005) Archaean atmospheric evolution: evidence from the Witwatersrand gold fields, South Africa. Earth-Science Reviews 70: 1-46
Frimmel HE, Nwaila GT (2020) Geologic evidence of syngenetic gold in the Witwatersrand goldfields, South Africa. In: Sillitoe RH, Goldfarb RJ, Robert F, Simmons SF (eds) Geology of the World’s Major Gold Deposits and Provinces. Special Publication 23. Society of Economic Geologists, Littleton, pp 645-668, doi: 10.5382/SP.23.31
Habashi F (1999) A Textbook of Hydrometallurgy. Metallurgie Extractive, 739 pp
Hallbauer DK, Maske S (eds) (1986) The mineralogy and geochemistry of Witwatersrand pyrite, gold, uranium, and carbonaceous matter. South Africa: Geological Society of South Africa
Hockaday L, McKechnie T, von Puttkamer MN, Lubkoll M (2020) The impact of solar resource characteristics on solar thermal pre-heating of manganese ores. In: Chen X and 10 coeditors (eds) Energy Technology 2020: Recycling, Carbon Dioxide Management, and Other Technologies, The Minerals, Metals & Materials Series. Springer, Cham, pp 3-13, doi: 10.1007/978-3-030-36830-2_1
Jones RT, Pawlik C (2019) Cobalt recovery from Southern African copper smelters. In: Proceedings of the 58th Annual Conference of Metallurgists (COM) Hosting the 10th International Copper Conference 2019, Vancouver, 18–21 August 2019
Minter WE, Goedhart ML, Knight JB, Frimmel, HE (1993) Morphology of Witwatersrand gold grains from the Basal Reef; evidence for their detrital origin. Economic Geology 88: 237-248
Nwaila GT and 5 coauthors (2020) Geometallurgical approach for impli-cations of ore blending on cyanide leaching and adsorption behaviour of Witwatersrand gold ores, South Africa. Natural Resources Research 29: 1007-1030, doi: 10.1007/s11053-019-09522-4
Parbhakar-Fox A, Baumgartner R (2023) Action versus reaction: how geometal-lurgy can improve mine waste manage-ment across the life-of-mine. Elements 19: 371-376
Pereira L, Schach E, Tolosana-Delgado R, Frenzel M (2023) All about particles: modelling ore behaviour in mineral processing. Elements 19: 359-364
Olsen SE, Tangstad M, Lindstad T (2007) Production of Manganese Ferroalloys. Tapir Akademisk Forlag, 247 pp
Souza Filho IR and 6 coauthors (2022) Green steel at its crossroads: hybrid hydrogen-based reduction of iron ores. Journal of Cleaner Production 340, 1-11, 10.1016/j. jclepro.2022.130805
Steenkamp JD, Chetty D, Singh A, Hockaday SAC, Denton GM (2020) From ore body to high temperature processing of complex ores: manganese—a South African perspective. JOM 72: 3422-3435, 10.1007/ s11837-020-04318-x
Tangstad M, Sibony M, Wasbø S, Tronstad R (2001) Kinetics of the pre-reduction of manganese ores. In: The Ninth International Ferroalloys Congress and the Manganese 2001 Health Issues Symposium: Official Proceedings, Quebec City, 3–6 June 2001, pp 202-207

