Glasses and Melts: Linking Geochemistry and Materials Science
Georges Calas, Grant S. Henderson, and Jonathan F. Stebbins – Guest Editors
Table of Contents
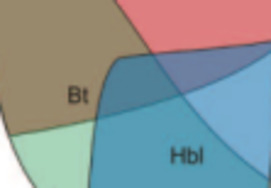
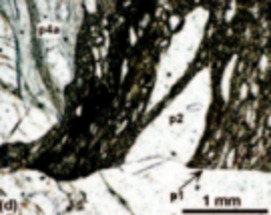
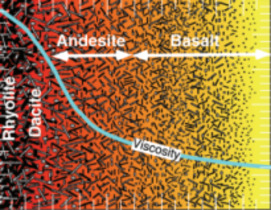
Geological interest in studying melts stems from early recognition that melts play a fundamental role in determining the physical and chemi- cal behaviour of magmas and magmatic processes. However, due to the inherent difficulties associated with working at high temperatures, much of the geological research over the last 30 years has used quenched melts or glasses as proxies for melts themselves. The assump- tion that the structure of the glass resembles that of the melt has been found to be good, at least at the temperature where the melt transforms to a glass. We will review how glass research has contributed to our understanding of melt structure and the behaviour of magmas. Emphasis is placed on elucidating the links between our knowledge of the atomic structure of melts and the macroscopic behaviour of magmas such as rheology, diffusion, trace element partitioning and redox behaviour.
- Glasses and Melts: Linking Geochemistry and Materials Science
- The Structure of Silicate Glasses and Melts
- Geochemical Aspects of Melts: Volatiles and Redox Behavior
- Transport Properties of Magmas: Diffusion and Rheology
- Dynamics of Magmatic Systems
- Structure–Property Relationships in Industrial and Natural Glasses
Activation Laboratories Ltd. (Actlabs)
Excalibur Mineral Corporation
Geological Society of London
Hudson Institute of Mineralogy
Materials Data (MDI)
Meiji Techno America
PANalytical
Rigaku
RockWare
v2n6 The Nuclear Fuel Cycle: Environmental Aspects
Guest editor: Rodney C. Ewing (University of Michigan)
Increasing concerns for the effects of global warming that result from rising greenhouse gas concentrations in the atmosphere have led to a reexamination, even enthusiasm, for nuclear power. Of all the current alternatives to fossil fuels, nuclear fission is the most important source of energy, accounting for 17 percent of the world’s electricity. In the United States, and indeed worldwide, Generation IV reactors and an Advanced Fuel Cycle Initiative are actively promoted, but major issues of nuclear waste management and disposal remain unanswered. This issue will focus on the impact of the nuclear fuel cycle on the environ- ment, particularly in terms of the materials that may be part of the waste streams.
- The Nuclear Fuel Cycle: A Role for Mineralogy and Geochemistry Rodney C. Ewing (University of Michigan)
- Uranium Mill Tailings: Geochemistry, Mineralogy, and Environmental Impact Abdesselam Abdelouas (Subatech Laboratory, Nantes)
- Spent Nuclear Fuel Jordi Bruno (Enviros, Barcelona) and Rodney C. Ewing (University of Michigan)
- Uranium Mineralogy and Neptunium Mobility Peter C. Burns and Amanda L. Klingensmith
- Nuclear Waste Glasses – How Durable? Bernd Grambow (Subatech Laboratory, Nantes)
- Ceramic Waste Forms for Actinides Gregory R. Lumpkin
- User Research Facilities in the Earth Sciences (February 2006 )
- Arsenic (April 2006)
- Water on Mars (June 2006)
- Early Earth (August 2006)
- Glasses and Melts: Linking Geochemistry and Materials Science (October 2006 )
- The Nuclear Fuel Cycle: Environmental Aspects (December 2006)