The Nuclear Fuel Cycle: Environmental Aspects
Rodney C. Ewing – Guest Editors
Table of Contents
Increasing concerns for the effects of global warming that result from rising greenhouse gas concentrations in the atmosphere have led to a reexamination, even enthusiasm, for nuclear power. Of all the current alternatives to fossil fuels, nuclear fission is the most important source of energy, accounting for 17 percent of the world’s electricity. In the United States, and indeed worldwide, Generation IV reactors and an Advanced Fuel Cycle Initiative are actively promoted, but major issues of nuclear waste management and disposal remain unanswered. This issue will focus on the impact of the nuclear fuel cycle on the environ- ment, particularly in terms of the materials that may be part of the waste streams.
- The Nuclear Fuel Cycle: A Role for Mineralogy and Geochemistry
- Uranium Mill Tailings: Geochemistry, Mineralogy, and Environmental Impact
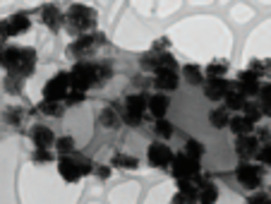
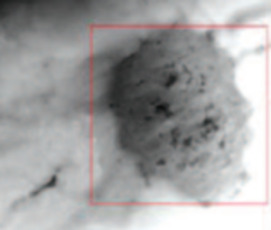
- Spent Nuclear Fuel
- Uranium Mineralogy and Neptunium Mobility
- Nuclear Waste Glasses – How Durable?
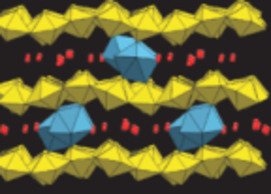
- Ceramic Waste Forms for Actinides
Activation Laboratories (Actlabs)
Australian Scientific Instruments (ASI)
Excalibur Mineral Corporation
Hudson Institute of Mineralogy
Materials Data (MDI)
Meiji Techno America
MIT Press
PANalytical
Photo-Atlas of Minerals
Rigaku
RockWare
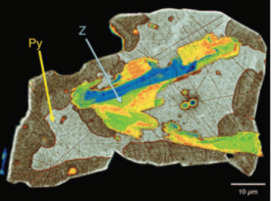
v3n1 Zircon – Tiny but Timely
Guest editor: Simon L. Harley (University of Edinburgh) and Nigel M. Kelly (University of Edinburgh)
Where would Earth science be without zircon? As Earth’s timekeeper, zircon has proven to be a remarkable and versa- tile mineral, providing insights into deep time and ancient Earth processes. However, there is still much to learn about Earth’s history from zir- con and its behaviour. Zircon cannot be treated simply as a passive “safehouse” of stored isotopic and chemical infor- mation but must instead be interpreted carefully, in its petrological, mineralogical, and geological contexts, and in the light of all possible lines of evidence. Zircon has been a wonderful servant in our quest to unravel the his- tory of the Earth—and has so much more to offer as we unlock the secrets of its chemical and physical responses to the processes operating in the Earth.
- Zircon Tiny but Timely Simon L. Harley (University of Edinburgh) and Nigel M. Kelly (University of Edinburgh)
- Zircon as a Monitor of Crustal Growth Erik E. Scherer (University of Münster), Martin J. Whitehouse (NORDSIM, Sweden) and Carsten Münker (Universität Bonn)
- Zircon Behaviour and the Thermal Histories of Mountain Chains Simon L. Harley (University of Edinburgh), Nigel M. Kelly (University of Edinburgh) and Andreas Möller
- Zircon Behaviour in Deeply Subducted Rocks Daniela Rubatto (Australian National University) and Jörg Hermann (Australian National University)
- Rare Earth Element Behavior in Zircon–Melt Systems John M. Hanchar (Memorial University of Newfoundland) and Wim van Westrenen Vrije Universiteit)
- Re-equilibration of Zircon in Aqueous Fluids and Melts Thorsten Geisler (Universität Münster), Urs Schaltegger (Université de Genève) and Frank Tomaschek (Universität Münster)
- Hydrothermal Zircon Urs Schaltegger (Université de Genève)
- User Research Facilities in the Earth Sciences (February 2006 )
- Arsenic (April 2006)
- Water on Mars (June 2006)
- Early Earth (August 2006)
- Glasses and Melts: Linking Geochemistry and Materials Science (October 2006 )
- The Nuclear Fuel Cycle: Environmental Aspects (December 2006)