Iron in Earth Surface Systems
Kevin G. Taylor and Kurt O. Konhauser – Guest Editors
Table of Contents
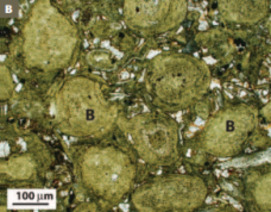
Iron is the fourth most abundant element at the Earth’s surface. As an essential nutrient and electron source/sink for the growth of microbial organisms, it is metabolically cycled between reduced and oxidized chem ical forms. This flow of electrons is invariably tied to the reaction with other redox sensitive elements, including oxygen, carbon, nitrogen, and sulfur. The end result of these interac tions is that iron is intimately involved in the geochemistry, mineralogy, and petrology of modern aquatic systems and their associated sediments, particulates, and pore waters. In the geological past, vast iron sediments, the so called banded iron formations, suggest that iron played an even greater role in marine geochemistry, and these deposits are now being used as proxies for understanding the chemical composition of the ancient oceans and atmosphere. This issue will explore not only the modern expression of iron cycling but also its record in Earth’s history.
- Iron in Earth Surface Systems: A Major Player in Chemical and Biological Processes
- IRON IN MICROBIAL METABOLISMS
- Geomicrobiology of Iron in Extreme Environments
- Iron Transport from the Continents to the Open Ocean: The Aging–Rejuvenation Cycle
- Ferruginous Conditions: A Dominant Feature of the Ocean through Earth’s History
- Iron Minerals in Marine Sediments Record Chemical Environments
- Iron Ore Deposits Associated with Precambrian Iron Formations
10th International Kimberlite Conference
Bruker
CAMECA
Cosmochemistry Illustrated
Excalibur Mineral Corporation
FIMIN ESF Research Network Program
PSRD Discoveries
RockWare
Savillex
SPECTRO
v7n3 GLOBAL WATER SUSTAINABILITY
Guest editor: Janet Hering, Chen Zhu and Eric H. Oelkers
The term water resources refers to natural waters (vapor, liquid, or solid) that occur on the Earth and that are of potential use to humans. These resources include oceans, rivers, lakes, groundwater, and glaciers. The Earth has plenty of water, over 1.4 × 109 km3. However, 97% of global water is saline sea water. Of the 3% that is freshwater, nearly 70% is locked in the polar icecaps and gla ciers. The majority of nonglacier freshwater is groundwater (98%). Surface freshwater (rivers and lakes), which has historically served most human needs, constitutes only a small fraction of the Earth’s water resources. Water interacts with minerals, soils, sediments, and rocks, and hence studies of Earth materials have a direct bearing on water resources. Studies of the acquisi tion, mobility, and fate of elements and isotopes in water provide valuable sig natures for tracking water cycles at regional and global scales and are essential for the development of remediation technologies for contaminated water.
- Water: Is There a Global Crisis? Eric H. Oelkers (University College London), Janet G. Hering (Eawag), and Chen Zhu (Ohio State University)
- Water and Sanitation in Developing Countries: Geochemical Aspects of Quality and Treatment Richard B. Johnston, Michael Berg, C. Annette Johnson, Elizabeth Tilley, and Janet Hering (Eawag)
- Hydrogeochemical Processes and Controls on Water Quality and Water Management Chen Zhu (Ohio State University) and Franklin W. Schwartz (Ohio State University)
- Groundwater: A Resource in Decline Franklin W. Schwartz (Ohio State University) and Motomu Ibaraki (Ohio State University)
- Water Management Challenges Associated with the Production of Shale Gas by Hydraulic Fracturing Kelvin B. Gregory (Carnegie Mellon University) and David A. Dzombak (Carnegie Mellon University)
- Water Conservation, Efficiency, and Reuse Henry Vaux Jr. (University of California–Riverside)
- Cosmochemistry (February 2011)
- Iron in Earth Surface Systems (April 2011)
- Global Water Sustainability (June 2011)
- When the Continental Crust Melts (August 2011)
- Tourmaline (October 2011)
- Mine Wastes (December 2011)
Download 2012 Thematic Preview