Minerals, Microbes, and Remediation
Anhuai Lu and Hailiang Dong – Guest Editors
Table of Contents
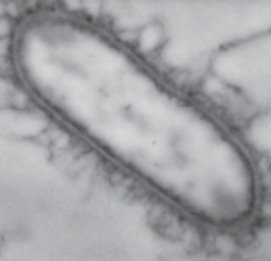
Studies of mineral–microbe interactions lie at the heart of the emerging field of geomicrobiology, because minerals are the fundamental earth materials with which microbes interact. microbes are found in a number of the earth’s extreme environments and also in extraterrestrial materials. In spite of the diverse geolog ical environments in which microbes are found and the various approaches taken to study them, a common thread—min eral–microbe interactions—connects all these environments and experimental approaches and places them under the same umbrella: geomicrobiology. minerals provide microbes with energy and living habitats, and microbes impact mineral weathering and diagenesis. the recognition of mineral–microbe interac tions has revived the classical discipline of mineralogy, and microbes discovered in various habitats have provided micro biologists with unique opportunities for study. this issue considers microbially mediated mineral dissolution, precipitation, and transformation, and the syn ergistic relation between minerals and microbes for energy acquisition. these interactions have important implications for contaminant remediation.
- Mineral–Microbe Interactions and Implications for Remediation
- Minerals as Substrates for Life: The Prokaryotic View
- Remediation of Chromium and Uranium Contamination by Microbial Activity
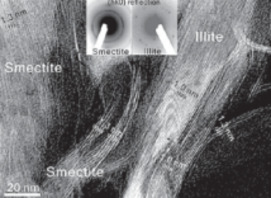
- Clay–Microbe Interactions and Implications for Environmental Mitigation
- Microbial Oxidation of Sulfide Tailings and the Environmental Consequences
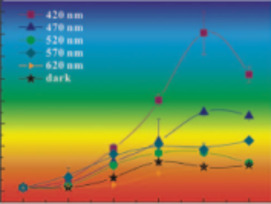
- Interactions between Semiconducting Minerals and Bacteria under Light
Actlabs
AHF Analysentechnik
Bruker
Bruker Nano GmbH
CAMECA
Excalibur Mineral Corporation
FEI
McCrone Microscope and Accessories
Pan Stanford Publishing
Geochemist’s Workbench
Rigaku
Rocks & Minerals
Savillex
SPECTRO
Thermo Scientific
v8n3 FUKUSHIMA DAI-ICHI: ONE YEAR LATER
Guest Editors: Takashi Murakami (University of tokyo) and Rodney C. Ewing (University of michigan)
On march 11, 2011, an earthquake and tsunami hit Japan, killing more than 20,000 persons, displacing tens of thousands, and causing havoc in the infra structure and economy of the country. In the aftermath of this tragedy, the cooling systems of three of the operating reactors at the Fukushima Dai ichi nuclear power station failed and meltdown of the reactor cores occurred. Over the following days, a series of hydrogen gas explosions took place. radionuclides (mainly 131 I and 137Cs) were released to the atmosphere and transported over many tens of kilometers from the site, contaminating soil and water. seawater was used to cool the damaged reactor cores, and water contaminated with radio activity was released to the ocean. Considerable amounts of used fuel were stored in nearby pools, and with the loss of water, the pools contributed to the release of radioactivity. One year after the tragedy at Fukushima, this issue of Elements provides a summary of what is known about the environmental impact of this nuclear accident.
- Fukushima Daiichi More Than One Year Later By Rodney C. Ewing (Stanford University) and Takashi Murakami (Japan Atomic Energy Agency)
- The 2011 Tohoku Earthquake By Jeroen Ritsema (University of Michigan), Thorne Lay (University of California–Santa Cruz), and Hiroo Kanamori (California Institute of Technology)
- Examining the Nuclear Accident at Fukushima Daiichi By Edward D. Blandford (Stanford University), Waturu Mizumachi (Japan Nuclear Energy Safety Organization), and Joonhong Ahn (University of California, Berkeley)
- Atmospheric Dispersion and Deposition of Radionuclides from the Fukushima Daiichi Nuclear Power Plant Accident By Anne Mathieu (Institute of Radiation Protection and Nuclear Safety) and coauthors
- Land-Surface Contamination by Radionuclides from the Fukushima Daiichi Nuclear Power Plant Accident By Naohiro Yoshida (Tokyo Institute of Technology) and coauthors
- Oceanic Dispersion Simulations of 137Cs Released from the Fukushima Daiichi Nuclear Power Plant By Yukio Masumoto (Japan Agency for Marine-Earth Science and Technology)
- Interactions between Nuclear Fuel and Water at the Fukushima Daiichi Reactors By Bernd Grambow (Université de Nantes) and Christophe Poinssot (CEA, France)
- Impact! (February 2012)
- Minerals, Microbes, and Remediations (April 2012)
- Fukushima Daiichi (June 2012)
- Granitic Pegmatites (August 2012)
- Rare Earth Elements (October 2012)
- Urban Geochemistry (December 2012)
Download 2012 Thematic Preview