Nitrogen and Its (Biogeocosmo) Chemical Cycling
Gray E. Bebout, Marilyn L. Fogel, and Pierre Cartigny – Guest Editors
Table of Contents
Nitrogen is the most abundant element in Earth’s atmosphere and a key compo- nent of the biosphere. It is also a critical part of the surface/near-surface cycling of nutrients, thus directly impacting our lives. Changes in the biogeochemical cycling of nitrogen through Earth’s history could reflect fundamental changes in its pathways from inorganic to biological reservoirs in response to change in the environment (e.g. oxygen fugacity in the atmosphere and oceans). Recognition of the importance of nitrogen to life on Earth, and likely elsewhere in the Solar System, has led to the mantra “Follow the Nitrogen” as one vehicle for focusing efforts in the search for extraterrestrial life. Nitrogen serves as a useful tracer of the transfer of “organic” signatures into the deep Earth (in records preserved in metamorphic and igneous rocks and in volcanic gases and rocks). It has been speculated that biological fi xation of nitrogen and storage in rapidly forming continental crust has led to drawdown of nitrogen from the early-Earth atmo-sphere, strongly influencing the chemical evolution of the atmosphere and related surface conditions.
- Nitrogen: Highly Volatile yet Surprisingly Compatible
- Stable Isotopes as Tracers of Anthropogenic Nitrogen Sources, Deposition, and Impacts
- Biogeochemical Cycling of Nitrogen on the Early Earth
- Nitrogen in the Silicate Earth: Speciation and Isotopic Behavior during Mineral–Fluid Interactions
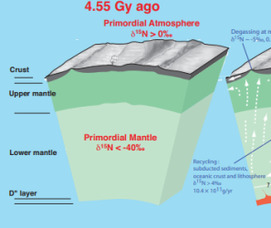
- Nitrogen Isotopes and Mantle Geodynamics: The Emergence of Life and the Atmosphere– Crust–Mantle Connection
- Nitrogen in Extraterrestrial Environments: Clues to the Possible Presence of Life
14th IAGOD Symposium
AHF Analysentechnik
Bruker
Bruker Nano
Cambridge University Press
Cameca
Elsevier (France)
FEI
Excalibur Mineral Corporation
Geochemist’s Workbench
JEOL
McCrone Microscope and Accessories
Rigaku
Savillex
SPECTRO
Thermo Scientific
TSI
v9n6 GARNET: COMMON MINERAL, UNCOMMONLY USEFUL
Guest Editors: Ethan F. Baxter (Boston University), Mark J. Caddick (Virginia Tech), and Jay J. Ague (Yale University)
Garnet is among the most studied—and most beloved—minerals, owing to its commonality in diverse geologic contexts, its often large euhedral crystals, its sometimes dazzling colors, and its propensity for preserving information about its growth history. Chemically zoned garnet represents a remarkable tool for deciphering metamorphic conditions and the evolving tectonic processes that drive garnet growth over many millions of years. In the deep Earth, garnet is a key rock-forming mineral, influencing the physical properties of the mantle and the composition of mantle-derived magmas. Garnet has been sought for ages as a semiprecious gemstone (the birthstone of January) and has been mined or synthesized (including nonsilicate garnet) for industrial purposes, including laser, magnetic, and ion-conductor technology. This issue of Elements will emphasize the most recent innovations in thermodynamic, geochemical, geochrono- logic, and industrial applications of garnet, while providing perspective on decades of garnet-related research.
- Garnet: Common Mineral, Uncommonly Useful Ethan F. Baxter (Boston University), Mark J. Caddick (Virginia Tech), and Jay J. Ague (Yale University)
- Garnet in the Earth’s Mantle Bernard J. Wood (University of Oxford) and Andrew K. Matzen (University of Oxford)
- Garnet: Witness to the Evolution of Destructive Plate Boundaries Mark J. Caddick (Virginia Tech) and Matthew J. Kohn (Boise State University)
- Garnet Geochronology: Timekeeper of Tectonometamorphic Processes Ethan F. Baxter (Boston University) and Erik E. Scherer (University of Münster)
- Metamorphism as Garnet Sees It: The Kinetics of Nucleation and Growth, Equilibration, and Diffusional Relaxation Jay J. Ague (Yale University) and William D. Carlson (The University of Texas at Austin)
- Garnet: A Key Phase in Nature, the Laboratory, and Technology Charles A. Geiger (Salzburg University)
- Garnet: From Stone to Star Laurence Galoisy
- One Hundred Years of Geochronology (February 2013)
- Serpentinites (April 2013)
- The Mineral-Water Interface (June)
- Continental Crust at Mantle Depths (August 2013)
- Nitrogen and Its (Biogeocosmo)Chemical Cycling (October 2013)
- Garnet: Common Mineral, Uncommonly Useful (December 2013)
Download 2013 Thematic Preview